
Steve Bittinger. Spider Web, Deua National Park, near Braidwood, NSW, Australia. Flickr.co, April 5, 2010.
Natural Fibers, spider webs, and hydrogen bonds
Hydrogen bonds are living malleable glue.
They help bring things together to create living systems. In this article, I will use the analogy of spider webs - a very common natural fiber - to highlight hydrogen bonds.
Natural fibers and DNA
Natural fibers, like hair, silk, wool, and Abaca fibers have long strings of amino acids, with each molecule attached to the next - like a chain of paper clips.
The amino acid molecules connect through strong structural bonds. Those bonds need enzymes to break them. The long chains of amino acids form microscopic threads.
Thicker strands form when those threads of amino acid chains connect to each other, like a stack of pliable railroad tracks. For example, each strand of human hair is made (among other things) of hundreds of those amino acid chains).
Connections between threads of amino acid chains include weak hydrogen bonds, like those dashed lines shown in the image on the right.
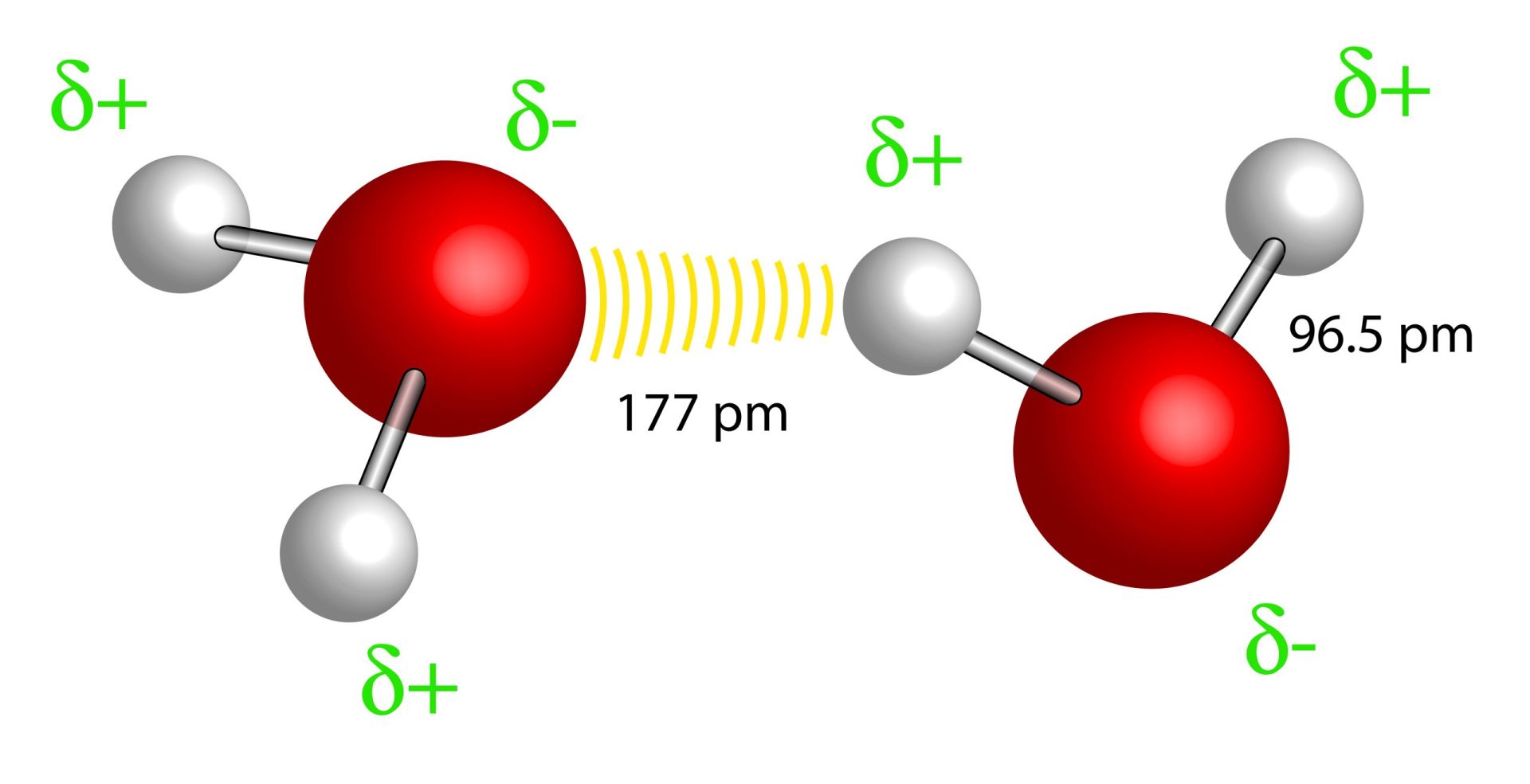
magnetix. hydrogen bridge bond between H2O molecules. Shutterstock.com, ID 351153710.
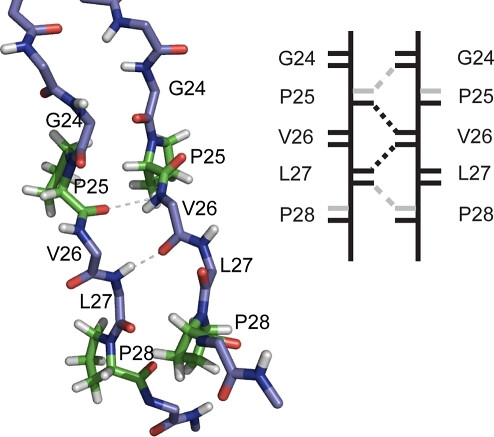
Thomas Brueckner. PLoS Comput Biol. 2009 Apr;5(4):e1000357. doi: 10.1371/journal.pcbi.1000357. Epub 2009 Apr 10.
Do all natural molecules have hydrogen bond connections? Yes, basically. Look at any molecule that has been made for a “living” purpose; these molecules will have some element of hydrogen bonding bringing them together:
The bark in the tree, the DNA in our cells, the mucus that we cough up, the tears that we shed!
Hydrogen bonds are the living glue that links us, one molecule at a time.

Ronald Tagra. Processing Abaca Fiber somewhere in Sogod, Southern Leyte. Abaca (aka Manila Hemp) is a kind of banana specie that bear less edible fruit but the trunk being harvested for its fiber. Flickr.com, Nov 22, 2012. Abaca is the strongest of natural fibers. The Philippines is the world’s largest source and supplier.
Living furniture glue
The easiest way to think of these living systems is through an analogy: thinking of sturdy molecular systems as furniture that is glued together.
Having hydrogen bonds is nature’s way of giving flexibility to structural elements.
It is certainly easier to separate two glued pieces of furniture at the glue joint by softening the glue with solvent than it is breaking down the wood and repairing that.
To take our furniture analogy one step further, having temporary bonds allows college kids to take the same furniture in their dorm room and create different arrangements that work for the roommates that have to share the living quarters.
The furniture structural elements have not changed; where and how you attach them has.
Temporary and moldable
Most importantly, hydrogen bonds get created virtually instantly when necessary.
Let’s take spider web silk as an example: the spider produces a mass of protein strands in its silk gland; these proteins are swimming in a soupy fluid in the spider’s silk-producing gland.
The protein strands are attached to each other only at one end, close to the opening of the gland. That allows the strands to stay unattached and floating free within the gland's fluid, like strands of pasta in a large pot of hot water.
When the spider wants to use the silk (either to escape or make a spider web) it attaches the first portion of the joined protein strands to a stationary object, say a tree branch.The strands then pass through an increasingly narrow spigot at the entrance of the silk gland.
What is the result? the protein filaments are squeezed together in the micro-hair-width spigot tunnel and the excess water is removed.

L Church "Golden Silk Spider Web - Nephila clavipes" Flickr - Photosharing! taken on July 11, 2009
A bunch of wet spaghetti pasta strands
This would be similar to grabbing a bunch of wet spaghetti pasta strands at one end with tweezers and then passing the lot through an ultra-thin funnel; the water would be squeezed out and the strands would stick together.
For the spider, passing the silk through the very narrow spigot encourages the virtually instantaneous formation of hydrogen bond links between the touching strands of molecules.
As the spider spools out the silk string, the protein strands zipper themselves together making one long silk thread.
Having the protein strands in a soluble state until they are ready to be used prevents congealed and tangled silk threads;
Snarled and knotted spider webs would bode poorly for survival for that species of spiders.

James Niland. "Spider busy pulling sticky thread". Flickr.com, April 10, 201
Speaking of cooking spaghetti, one way to test whether the pasta is cooked is to take one strand of spaghetti and throw it at the wall. If it sticks, the spaghetti is cooked! this is because enough of the starch has spread out/moisturized to allow hydrogen bonds to form between the molecules of the starch and those on the wall.
References
- Knight DP, Vollrath F. Spinning an elastic ribbon of spider silk. Philos Trans R Soc Lond B Biol Sci. 2002 Feb 28;357(1418):219-27.
- Keten S, Buehler MJ. Nanostructure and molecular mechanics of spider dragline silk protein assemblies. J R Soc Interface. 2010 Dec 6;7(53):1709-21.